
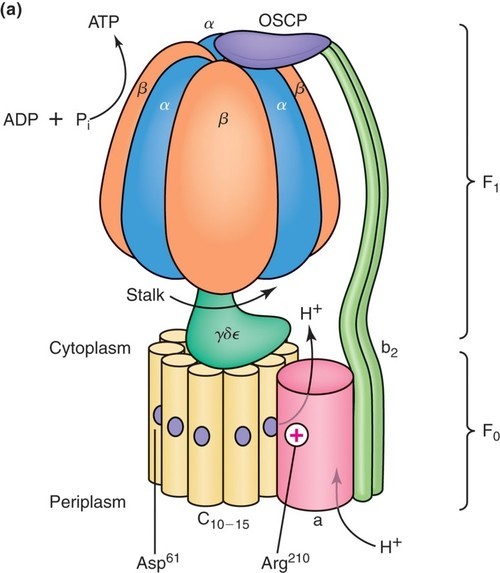
This could be easily demonstrated through the monitoring of oxygen consumption by mitochondria suspended in a buffered medium in an oxygen electrode apparatus (Figure 24.3, black line). Mitchell’s idea also neatly explained another aspect of ATP synthesis in mitochondria: coupling. The chemiosmotic theory readily explains the obligatory coupling seen between electron transfer and ATP synthesis: neither reaction occurs without the other.įigure 24.3 Monitoring oxygen consumption (black line) and ATP production (red line) demonstrates the coupling of redox reactions with ATP production. But instead of running on a circuit of electrons, as a battery does, the system runs on a proton circuit. Mitchell thought of the mitochondrion as being like a battery ( i.e., the electron transport chain) which charges up a capacitor ( i.e., the inner membrane). As protons flow back into the mitochondrial matrix down its concentration gradient, the energy of the electrochemical gradient is released and is used for the generation of ATP by the ATP synthase enzyme.Energy is stored as an electrochemical gradient (Proton motive force by analogy to an electromotive force in an electric circuit ).
#Proton gradient drives atp synthesis free
The free energy liberated by the redox reactions is used by the ETC to pump protons, moving H + from matrix to inter-membrane space.And he realized the chemical had to be the one associated with redox phenomena – the proton, because most redox reactions (electron transfers) are accompanied by proton transfers: i.e., NAD + + 2 e – + H + → NADH Mitchell saw that the connection between electron transport and ATP synthesis was not mediated by any mysterious chemical messenger (the old “chemical-coupling” idea), but depended on generation of a chemical gradient across the membrane. The “chemiosmotic hypothesis” not only explained mitochondrial ATP generation but also turned out to explain lots of other important processes (like photosynthesis and bacterial motility and many membrane transport phenomena).
This was until Peter Mitchell (Figure 24.2) proposed the chemiosmotic hypothesis (now the chemiosmotic theory) to explain the process. On the other hand, we have ATP synthesis, which involves forming a covalent chemical bond and has nothing to do with redox chemistry. Why? On the one hand, we have the electron transport chain, which involves redox reactions, not chemical bond formation. However, it was very hard to see how this system worked. How is this concentration of protons transformed into ATP? Figure 24.2 Peter Mitchellīy about 1960, scientists knew that mitochondria generated ATP using the free energy liberated by oxidation of reduced cofactors by the electron transport chain. (The same is true in the case of neurotransmission, where small numbers of K + and Cl – ions flow across gated ion channels in nerve cell membranes, resulting in large electrical impulses.) We can see that 200 kJ out of the 220kJ available from NADH oxidation is conserved in the proton gradient! The number of ions that need to be transported are small, but the energetic consequences are big. Total energy conserved in the proton gradient per NADH oxidized = 200 kJ Total # of H + pumped per mole of NADH oxidized through the ETC = 10

Δ Ψ : transmembrane difference in electrical potential Z: absolute value of the electrical charge of H + (1) ( ΔpH ≈ 0.75 pH unit)įree energy change for the transfer of 1 mole of H + across the inner mitochondrial membrane:Ĭ 2 & C 1 : concentration of H + outside and inside the IMM


 0 kommentar(er)
0 kommentar(er)
